The session focused on providing an update on the company’s clinical trial progress, tau pathology and therapeutic approaches to this important target in Alzheimer’s disease, and a detailed discussion between all attendees on how best to support the dissemination of research information to people with dementia, their families, and carers.
As the world awaits a significant therapeutic breakthrough for Alzheimer’s, post-diagnostic support for people with dementia is centre stage for this World Alzheimer’s Month. As part of this, being able to share important updates on LUCIDITY trial progress and outcomes is critical. TauRx invited attendees of the meeting to offer insights on what they understand the people with dementia want to learn about.
“Understanding what people need on a practical level following a diagnosis of Alzheimer’s is of utmost importance,” explains Dr Sonya Miller, Head of Medical Affairs and Clinical Safety Oversight Lead at TauRx. “A diagnosis is often the trigger for a complex system of therapy, care, and extended support necessary to allow people to live well with dementia and reduce the burden of the disease.
“Whilst we all continue to work towards a safe, effective, and affordable therapeutic answer to Alzheimer’s, the research industry must continue to find ways to help organisations such as Alzheimer Europe and its members to educate on progress in the field and share the latest information concerning clinical trials.”
“TauRx adds to this educational effort by sharing its decades of expertise in tau science and dedicated focus on neurodegenerative disease. But we must not make assumptions, and we learn more by asking questions in this forum to ensure we provide the right level of information and support that will benefit the patients and caregivers who need it most.”
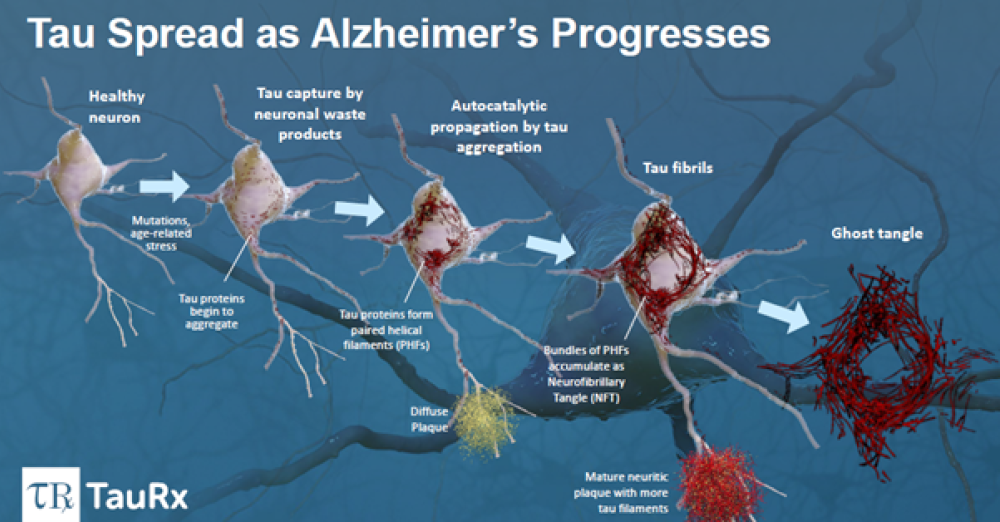
Aberdeen, Scotland and Singapore [14 September 2022] – TauRx Pharmaceuticals Ltd, the global leader in tau-based research in treatments for Alzheimer’s disease (AD)






